November 2018 –
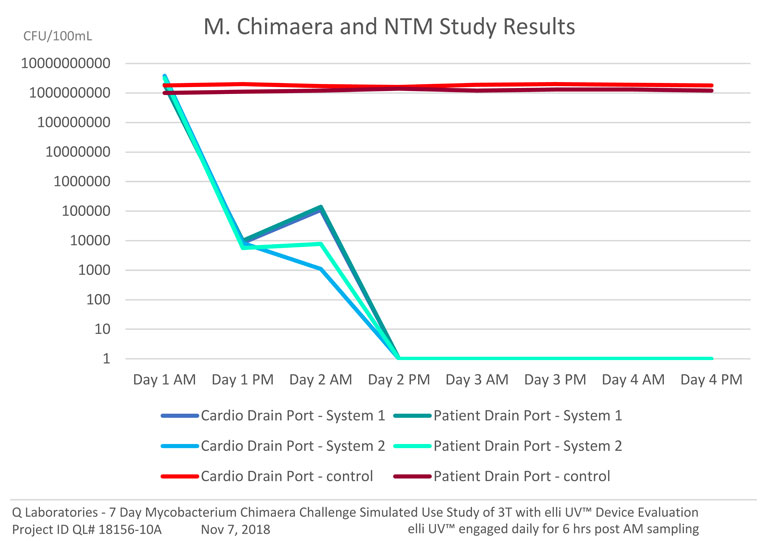
elli UV™ eliminated M. chimaera with an astounding Log 9.43 reduction in under 12 hours!
The Story of elli UV™
Some time ago we asked ourselves…Why is the use of UV-C as a solution to the ‘chimaera epidemic’ considered unrealistic? And why is everyone involved with this issue
skeptical about UV-C?

We needed to understand the answers to these questions before embarking upon our journey to a solution for M. chimaera plagued water found in Cardiac OR’s. What we discovered was:
- There are too many variables in relying on chemical disinfection alone, whether there is a protocol or not. The FDA has recognized this.
- Off the shelf UV-C cylinder designs were not maximizing UV-C light reflectivity.
- Almost EVERY attempt at using UV-C was unsuccessful due to the same misstep. Easy access to off-the- shelf UV-C stock lamps proved to be ineffective at targeting M. chimaera in high flowing water.
- No previous attempts that utilized UV-C ever successfully validated effectiveness with high flow water and M. chimaera.
- Until now, UV-C device manufacturers appeared reluctant to supply data supporting validation, which is a usual medical facility quality assurance expectation.
- None of the 35 UV-C manufacturing and supply companies contacted provided validation data.
- Most manufacturers offered stock UV-C lamps, referencing reports that cite 20 mJ lamps suffice to eliminate M. chimaera (Which hasn’t worked to efficiently target this robust bacterium in high flow water.)
- Only one company confirmed they could manufacture and validate our patented proprietary customized UV-accelerated- lamp-technology we knew would be crucial to our success and that is why we partnered with them.
-Available for Sale. Patent Pending Technology-
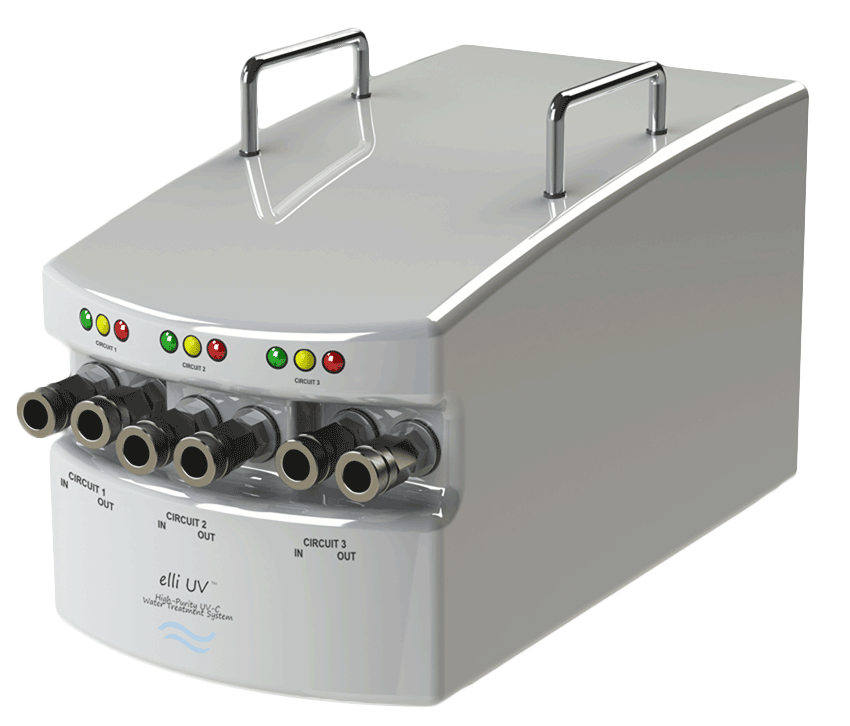
We succeeded. Introducing elli UV™
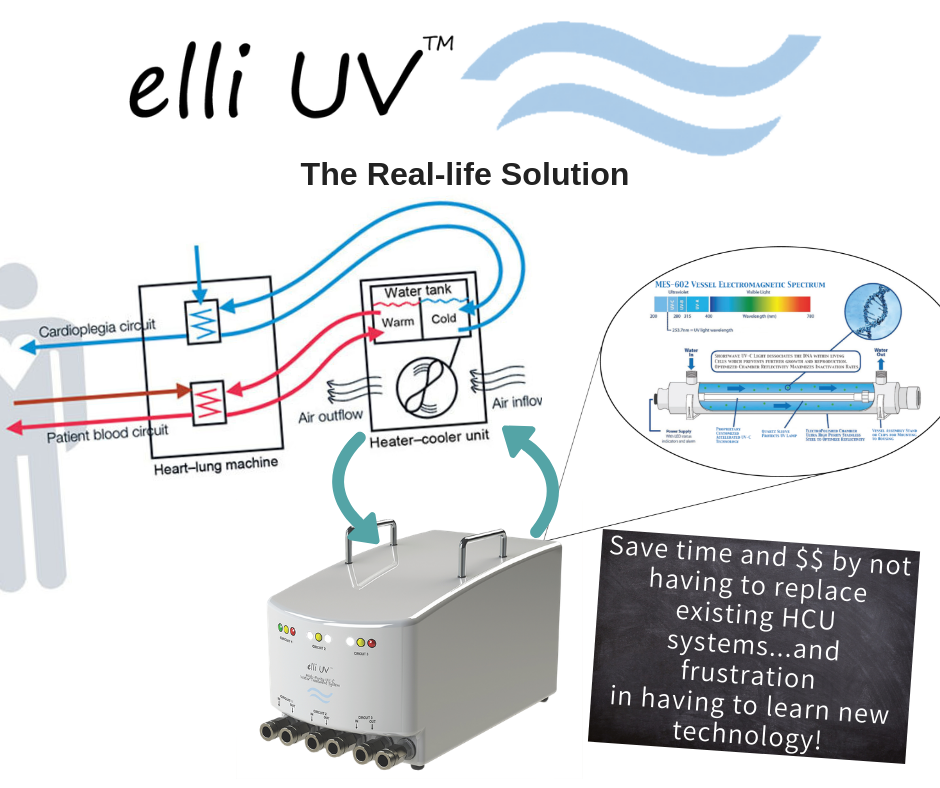
elli UV™ is the ONLY device Validated for the elimination of M. chimaera and other NTMs, as well as decrease HPCs in water within high flowing circuits.
What we did differently
We needed to ensure we didn’t duplicate the missteps of our predecessors. So, we consulted with microbiologists, micro-chemists, UV-C and flow dynamics engineers, health care professionals, infection control committees and medical device housing specialists to establish a development protocol.
• VALIDATED to an incredible Log 9.43 reduction of M. chimaera
• VALIDATED in 8L of high flow water, up to 20 LPM
• VALIDATED at 3 separate esteemed Canadian recognized testing facilities
• VALIDATED on on M. chimaera, other NTMs and HPCs
• PhD authored white paper from the VALIDATED test results
• Simplistic operation
• No effect on water flow pressure pre and post elli UV™
• No effect on water temperature
• Performs with 120V or 240V
• Virtually silent. Seen, but not heard.
-Available for Sale. Patent Pending Technology-
Test Results
Suspension Time Kill Study Results
Validated at 3 separate laboratories
June 2017 – Achieved Log 5 Reduction!
Michrochem Laboratory Study Identification Number NG8846 – Antibacterial Activity and Efficacy of MES-602 Water Treatment Device – ASTM International Method E2315 Modified for Device Assessment of Antimicrobial Activity using a Time-Kill Procedure June 13, 2017
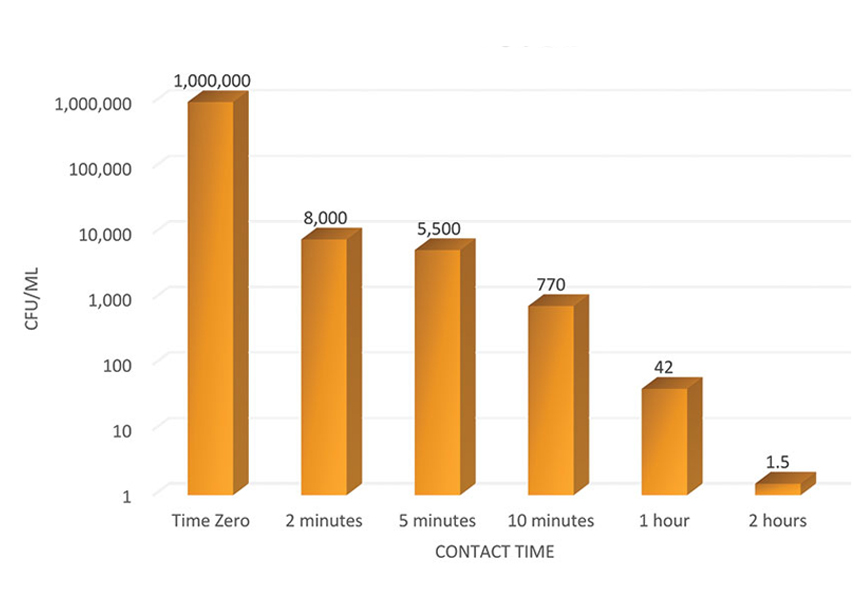
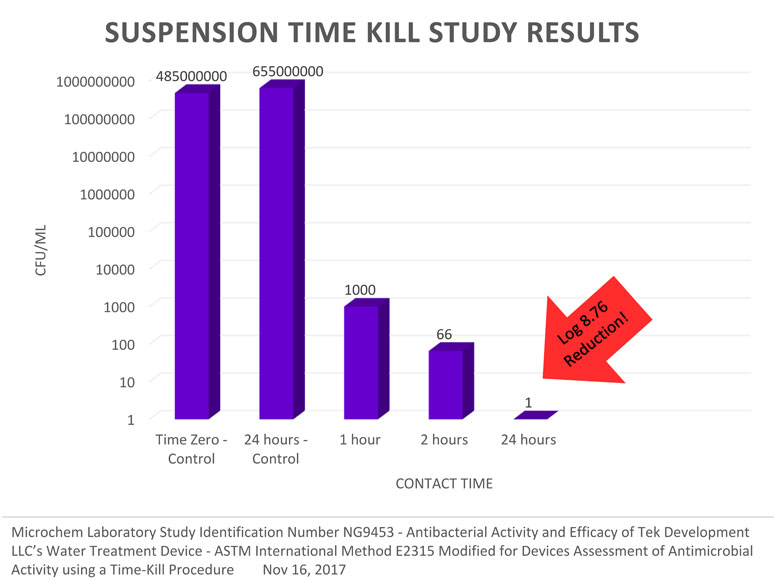
November 2017 – Achieved Log 8.76 Reduction!
After Some Enhancements
June 2018 – Cleans HCU in under 5 hours
Canadian hospital setting elli UV™ cleans a persistently contaminated HCU in under 5 hours.
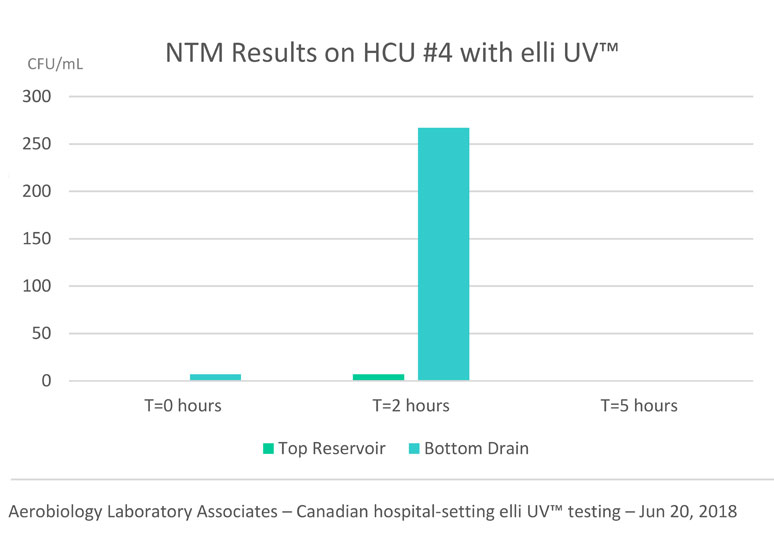
June 2018 – Cleans HCU in under 5 hours
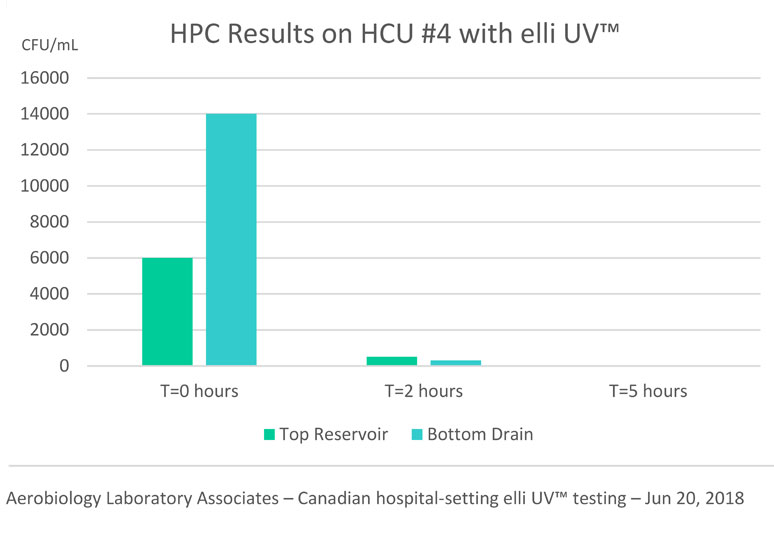
November 2018 – Dramatic Daily Reduction in HPC Codes
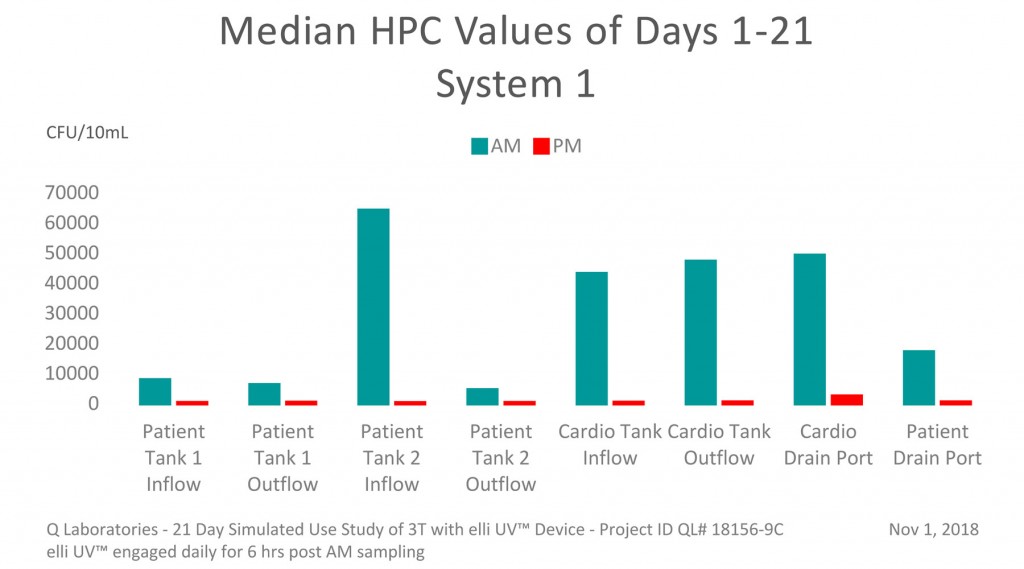
Using “in-line UV-C units [elli UV™] can provide real-time, continuous treatment of potentially contaminated waters circulating through heater-cooler pumps and other operating room equipment”
Luisa A. Ikner, Ph.D., Assistant Research Scientist – Water & Energy Sustainable Technology (WEST) Center, University of Arizona
-Available for Sale. Patent Pending Technology-